What are the differences between the Mutual Recognition Procedure and the Decentralized Procedure ?
From a legislative point of view, the mutual recognition procedure is defined by the Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the Community code relating to medicinal products for human use.
Directive 2004/27/EC subsequently laid the foundations for the decentralised procedure.
These two procedures are available for all marketing authorisation applications that do not fall within the mandatory scope of the centralised procedure. They are applicable whenever an applicant wishes to register its medicinal product in more than one Member State.
1/ The Mutual Recognition Procedure (MRP)
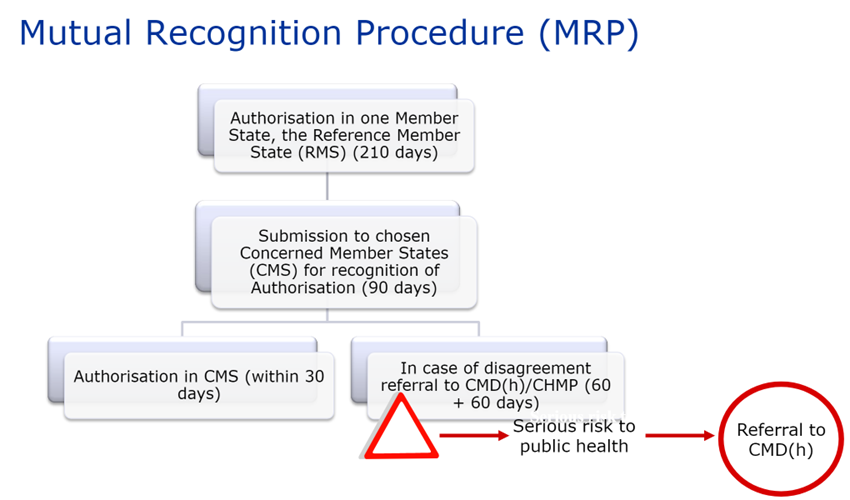
Article 28.2 of Directive 2001/83/EC, as amended, specifies the scope of this procedure: the principle is to extend a national MA already obtained in one of the EU Member States, to one or more other Concerned Member State(s) (or “CMS”) in which the laboratory wishes to market its product. The Reference Member State (or “RMS”), which granted the existing MA, manages the procedure.
Once the evaluation procedure has been completed, the MAs are issued by each of the competent authorities of the member states concerned.
Source EMA
After a possible upgrade of the MA dossier, the RMS sends the MA dossier and its assessment report, including the Summary of Product Characteristics (SPC), package leaflet and labeling, to the CMS 14 days before the start of the procedure.
The concerned Member States must then recognize the authorization already issued by the RMS within 90 days (without clock-stop).
The procedure may, however, end on Day 60 if the CMS has no further comments.
Once the MA (including the SPC, package leaflet and labeling) is recognized by the CMS, this recognition is followed by a 30-day national closing phase to issue the national MA. Procedures successfully closed are published on the CMDh website.
If a Member State has objections to recognizing the dossier’s assessment report, summary of product characteristics (SPC), package leaflet and labeling, on the grounds of a potentially serious risk to public health (as defined by Article 29(1) of Directive 2001/83/EC), the dossier is referred back to the CMDh for further discussion. This procedure lasts 60 days. If the CMDh is unable to reach a decision within 60 days, the application is sent to the CHMP for arbitration (art. 29(4) of Directive 2001/83/EC). This procedure also lasts 60 days.
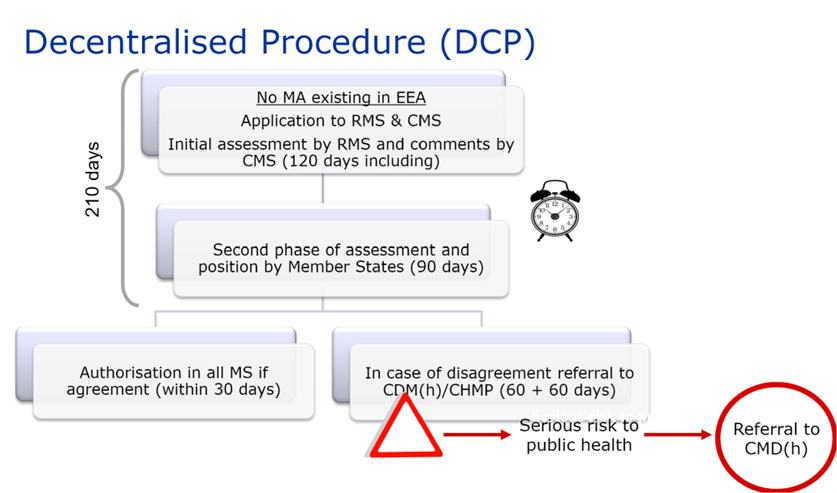
2/ The decentralized procedure (DCP)
This procedure differs from the MRP in two main points :
- No marketing must first have been granted in the EU,
- The dossier is submitted simultaneously in all Member States.
In this case, the laboratory requests a member state to act as reference state (“RMS”) in order to carry out the evaluation, among the states where it wishes to obtain authorization for its medicinal product.
Source EMA
* EEA = European Economic Area
MA = Marketing authorization
The RMS draws up a preliminary assessment report on the dossier submitted and the draft SPC, package leaflet and labeling.
This report is submitted to the CMS and the applicant for comments on Day 70 of the procedure.
On Day 105 of the procedure, the clock stops to allow the applicant to submit answers to the questions raised by the Member States at the end of phase 1.
When the answers have been submitted, the clock starts again on Day 106, and on Day 120 of the procedure, the RMS circulates at the same time an update of all documents (assessment report, SPC, package leaflet and labeling) to the applicant and the CMS.
A second phase of Questions and Answers begins, and the procedure may be closed on Day 150 if all comments have been resolved.
Otherwise, a new 60-day phase begins to finalize outstanding issues.
The DCP therefore lasts a maximum of 210 days. This period is followed by a 30-day national closing phase to issue the national MA. Procedures successfully closed are published on the CMDh website.
As with the MRP, if there is no consensus between member states, the dossier is referred back to the CMDh for further discussion. This procedure lasts 60 days. If the CMDh is unable to reach a decision within 60 days, the application is sent to the CHMP for arbitration (art. 29(4) of Directive 2001/83/EC). This procedure also lasts 60 days.
3/ To sum up:
| MRP | DCP |
| Existing initial national MA | No MA granted in the EU |
| No choice of the RMS (national MA already existing th the EU) | The choice of the RMS is up to the applicant |
| Request a recognition by the other Member States | Simultaneous application to all member states: the RMS evaluates the dossier for the first time (as for the CMS) |
| Only one evaluation phase | Two evaluation phases |
| Decision within 90 days, or up to 150 days in the event of arbitration by the CMDh if no consensus can be reached between Member States. | Decision in 210 days (excluding clock-stop period), or up to 270 days in the event of arbitration by the CMDh if no consensus can be reached between Member States. |
| MA Dossiers identical in all member states | |
| Principle of recognition of the evaluation of the Reference Member State (RMS) by the other Member States concerned (CMS) | |
| The choice of the States involved in these procedures is up to the applicant | |
| National closing phase of 30 days planned to issue the national MA | |
| A European Public Assessment Report (“PAR”) for each medicinal product approved via the MRP/DCP is published in the directory « Mutual Recognition Index » by the RMS on the HMA website. | |
ATESSIA supports laboratories throughout the registration process: from the registration strategy to the draft and submission of the marketing authorization applications.
Article written by Mathilde ISRAEL, Regulatory Affairs and External Communication Advisor.