The new regulation (EU) 2017/745 introduces new requirements to enhance the safety of patients and users. One of the novelties of this new regulation is the creation of a European database dedicated to information on medical devices called EUDAMED.
This database will allow:
– Increased transparency of information on medical devices with public access.
– Better coordination between Member States in the post-market surveillance of medical devices.
EUDAMED is a secure platform used to collect and share data related to medical devices placed on the European Union market, as well as those undergoing clinical investigation.
The regulation introduces new requirements for the various actors involved in EUDAMED.
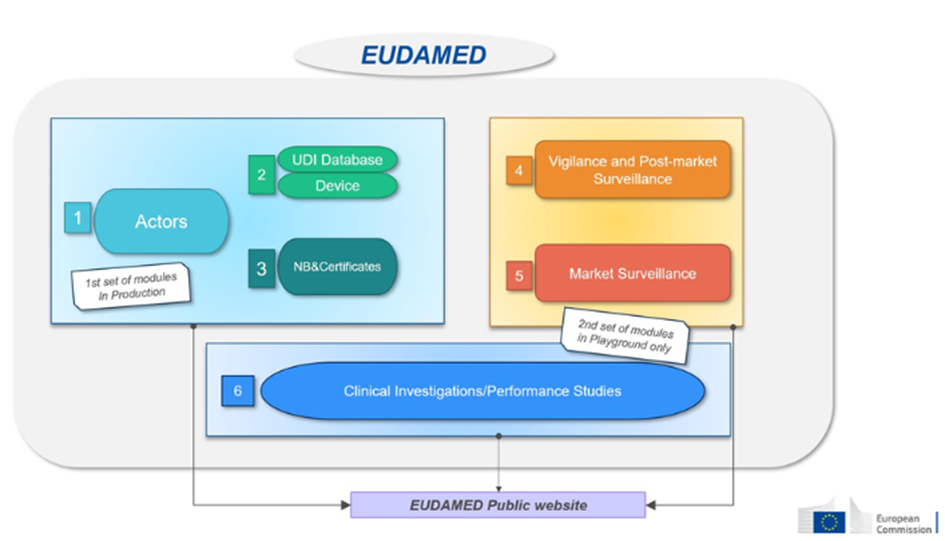
This database will consist of six interconnected modules:
| Module : | Who needs to record information? | Accessible to the public | |
| 1-Actors | Economic operators must register as actors in EUDAMED and provide the required information. | – EU and third-country manufacturers, – Authorized representatives, – System/procedure pack producers, – Importers. | Available on a voluntary basis since December 2020 and will be mandatory from Q1 2026. |
| 2-Devices | Manufacturers must submit the basic-UDI and information of all devices they place on the EU market into EUDAMED. | – Only manufacturers. Registration of medical devices under MDR. No obligation for legacy devices (if registered in EUDAMED, a new registration will be required for products under MDR, considered as new products). | Available on a voluntary basis since October 2021 and will be mandatory from Q1 2026. |
| 3- Notified Bodies (NB) and Certificates | . Notified bodies must register in EUDAMED all information regarding issued, suspended, reinstated, withdrawn, or refused certificates and other restrictions imposed on these certificates. This information is accessible to the public. | – Notified Bodies. | Available on a voluntary basis since October 2021 and will be mandatory from Q1 2026. |
| 4-Vigilance | Module dedicated to all vigilance and post-market surveillance reports. – Safety information (Field Safety Notice, FSN), – Field Safety Corrective Action (FSCA), – Investigation report of incident causes and corrective measures (MIR), – Trend report, – Periodic Safety Update Report (PSUR). | – Manufacturer. | Will be mandatory from Q3 2026. |
| 5- Market Surveillance | Coordination of market surveillance actions between the various competent authorities. | – Competent authority only. | Will be mandatory from Q1 2026. |
| 6- Clinical Investigation/Performance Studies (CI/PS): | This module concerns the registration of clinical investigations (MD) and performance studies (IVD). – Clinical investigation report and summary, – Serious adverse event during clinical investigations. | – Sponsor. | Not yet available. |
Source : European commission
And the Distributors?
The MDR imposes no requirements on distributors regarding EUDAMED. They have no secure access to EUDAMED and only have public access. However, some countries may set additional requirements, such as France, which requires distributors to register via the ANSM form.
EUDAMED Deployment Schedule
In October 2019, the European Commission announced a two-year postponement of EUDAMED’s launch to May 2022.
Some modules are already available and can be used voluntarily. A roadmap project was released on July 10, 2024, indicating a full deployment of EUDAMED scheduled for the second quarter of 2027. The dates present in the EUDAMED roadmap are provisional and in “Draft” mode. No dates are official at this stage.
Recently, on January 21, 2024, the European Commission published a proposal to amend regulation (EU) 2017/745 on medical devices (MDR) and regulation (EU) 2017/746 on in vitro diagnostic medical devices (IVDR) concerning the gradual deployment of EUDAMED. This proposal suggests a gradual implementation of EUDAMED modules once validated, potentially starting in Q4 2025. This proposal will need to be adopted and published in the Official Journal by May 2024.
Useful Documents and Guides:
– EUDAMED Release Note
– EUDAMED Roadmap

