Our latest News & Events
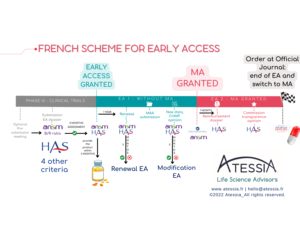
What are the mechanisms for early and compassionate access in France?
Atessia supports its clients daily in the practical modalities of implementing the French early and compassionate access system, whose subtleties require some explanations. On July

What are the differences between the Mutual Recognition Procedure and the Decentralized Procedure ?
From a legislative point of view, the mutual recognition procedure is defined by the Directive 2001/83/EC of the European Parliament and of the Council of

What about the classification of Marketing Authorization variations?
What about the classification of Marketing Authorization variations? When a holder wishes to register a medicine in a country, he submits a marketing authorization application

ATESSIA Life Science Advisors, a Catalyst for Regulatory Innovation: Collaborating with UBAQ, ATESSIA Sets a New Standard to Facilitate the Management of Regulatory Challenges in the Health Sector
An alliance capable of moving mountains. ATESSIA and UBAQ jointly pave the way for health sector players. The health sector, constantly evolving and in the

What Are the New Rules for Influencers on Social Networks?
Law No. 2023-451 dated June 9, 2023, which aims to regulate commercial influence and combat abuses by influencers on social media platforms, not only defines

What are the possible interactions between health industry players and healthcare professionals (HCPs) in France?
Promotional visit for medicinal products in France refers to promotional interactions with healthcare professionals (HCPs) conducted by authorized collaborators from the pharmaceutical industry. The structural

The Chief Pharmaceutical Officer
The Chief Pharmaceutical Officer also called Responsible Pharmacist (RP) is a key role , essential to the organization of any pharmaceutical laboratory involved in the

The serialisation system
Securing the distribution of medicines represents an unprecedented challenge for public health. Although France has always benefited from a particularly secure drug distribution system and

The APQR review “à la française”
What is an APQR? Both US and EU Good Manufacturing Practices (GMP) require manufacturers of all authorized medicinal products to perform Annual Product Quality Reviews
